Directors Dealings
Directors' Dealings
Personal trades by the Management Board
and Supervisory Board
According to article 19 of Market Abuse Regulation, persons with managerial responsibilities, as well as persons closely related to them, are required to report the purchase and sale of Company shares, or other financial instruments relating thereto, to the German Federal Financial Supervisory Authority (BaFin) and to the Company if the total value of such transactions effected within a calendar year is equal to or more than EUR 5,000.
Such transactions are, in accordance with German law, promptly published by Formycon upon such notification.
General information on the following managers transactions announcements
according to article 19 MAR
Description of the financial instrument: Share
Name of issuer: Formycon AG
ISIN of the financial instrument: DE000A1EWVY8
Legal Entity Identifier (LEI) Code: 39120005TZ76GQOY8Z19
| Date | Name | Position | Transaction | Volume | Price in € | Volume in € | Publication |
|---|---|---|---|---|---|---|---|
| 02/21/2025 | Dr. Andreas Seidl | Executive Board Member (CSO) | Purchase | 160 | 30 | 4,800.00 | Download |
| 04/25/2024 | Nicola Mikulcik | Executive Board Member (CBO) | Purchase | 1,200 | 40 | 48,000.00 | Download |
| 04/22/2024 | Dr. Stefan Glombitza | Executive Board Member (CEO) | Purchase | 2,340 | 39 | 91,260.00 | Download |
| 04/22/2024 | Dr. Stefan Glombitza | Executive Board Member (CEO) | Purchase | 160 | 38.65 | 6,184.00 | Download |
| 08/30/2022 | Dr. Stefan Glombitza | Executive Board Member (CEO) | Sale | 15,000 | 76.48 | 1,147,200.00 | Download |
| 08/04/2022 | Dr. Stefan Glombitza | Executive Board Member (CEO) | Purchase | 20,000 | 19.46 | 389,200.00 | Download |
| 03/03/2021 | Dr. Stefan Glombitza | Executive Board Member (COO) | Sale | 15,000 | 64.9 | 973,500.00 | Download |
| 03/03/2021 | Dr. Carsten Brockmeyer | Executive Board Member (CEO) | Sale | 7,500 | 64.9 | 486,750.00 | Download |
| 03/03/2021 | Dr. Nicolas Combé | Executive Board Member (CFO) | Sale | 15,000 | 64.9 | 973,500.00 | Download |
| 02/03/2021 | Dr. Stefan Glombitza | Executive Board Member (COO) | Purchase | 15,000 | 19.46 | 291,900.00 | Download |
| 02/03/2021 | Dr. Carsten Brockmeyer | Executive Board Member (CEO) | Purchase | 7,500 | 20.7 | 155,250.00 | Download |
| 02/03/2021 | Dr. Nicolas Combé | Executive Board Member (CFO) | Purchase | 15,000 | 20.7 | 310,500.00 | Download |
| 11/14/2019 | Peter Wendeln (Wendeln & Cie. KG) | Member of the Supervisory Board | Sale | 72,000 | 30.9 | 2,224,800.00 | Download |
| 07/23/2019 | Dr. Nicolas Combé | Executive Board Member (CFO) | Others | 25,000 | 5.52 | 138,000.00 | Download |
| 07/23/2019 | Dr. Carsten Brockmeyer | Executive Board Member (CEO) | Others | 25,000 | 5.52 | 138,000.00 | Download |
| 06/26/2019 | Peter Wendeln (Wendeln & Cie. KG) | Member of the Supervisory Board | Purchase | 577,397 | 29.9 | 17,264,170.30 | Download |
| 06/05/2019 | Peter Wendeln (Wendeln & Cie. KG) | Member of the Supervisory Board | Others | Not Quantifiable | Not Quantifiable | Not Quantifiable | Download |
| 08/10/2018 | Dr. Nicolas Combé | Executive Board Member (CFO) | Sale | 25,000 | 33.48 | 837,000.00 | Download |
| 08/10/2018 | Dr. Carsten Brockmeyer | Executive Board Member (CEO) | Sale | 25,000 | 33.48 | 837,000.00 | Download |
| 07/21/2018 | Dr. Nicolas Combé | Executive Board Member (CFO) | Purchase | 25,000 | 7.59 | 189,750.00 | Download |
| 07/21/2018 | Dr. Carsten Brockmeyer | Executive Board Member (CEO) | Purchase | 25,000 | 7.59 | 189,750.00 | Download |
| 09/05/2017 | Dr. Nicolas Combé | Executive Board Member (CFO) | Sale | 15,000 | 31.6 | 474,000.00 | Download |
| 09/05/2017 | Dr. Carsten Brockmeyer | Executive Board Member (CEO) | Sale | 15,000 | 31.6 | 474,000.00 | Download |
| 07/14/2017 | Dr. Nicolas Combé | Executive Board Member (CFO) | Purchase | 15,000 | 4.33 | 64,950.00 | Download |
| 07/14/2017 | Dr. Carsten Brockmeyer | Executive Board Member (CEO) | Purchase | 15,000 | 4.33 | 64,950.00 | Download |
Voting Rights Notifications
Voting Rights Notifications
Notifications pursuant to Sections 33 et seq. of the
German Securities Trading Act (WpHG)
Under the German Securities Trading Act (WpHG), the shareholders of a German listed company are obliged to inform the Company and the German Federal Financial Supervisory Authority (BaFin) of any changes in voting rights in Formycon AG if they reach, exceed or fall below the statutory thresholds. The voting rights notifications of Formycon AG are shown in the table below.
We kindly request shareholders who are obliged to submit voting right notifications according to §§ 33ff of the German Securities Trading Act (Wertpapierhandelsgesetz – WpHG) to send their notification in a readable version (pdf) and the XML file and any annexes for the publication of the notification of voting rights in writing to the e-mail address provided below.
| Date | Notifying party | Type of notification | Publication |
|---|---|---|---|
| 13.11.2024 | Klaus Röhrig | Stimmrechtsmitteilung | Download |
| 13.11.2024 | Florian Schuhbauer | Stimmrechtsmitteilung | Download |
| 11/11/2024 | Thomas Peter Maier | Voting Rights Notification | Download |
| 11/11/2024 | Peter Wendeln | Voting Rights Notification | Download |
| 11/11/2024 | Gedeon Richter | Voting Rights Notification | Download |
| 11/11/2024 | Detlef and Ursula Spruth | Voting Rights Notification | Download |
| 11/11/2024 | Stefan R. | Voting Rights Notification | Download |
IR Contact
Contact
Formycon in Dialogue
A key component of our philosophy is the dialogue with capital market players. The Formycon Investor Relations department will be happy to answer any questions or suggestions you may have.

Sabrina Müller
Director Investor Relations and Corporate Communications
T + 49 89 86 46 67 149 ・ F + 49 89 86 46 67 110
We kindly request shareholders who are obliged to submit voting right notifications according to §§ 33ff of the German Securities Trading Act (Wertpapierhandelsgesetz – WpHG) to send their notification in a readable version (pdf) and the XML file and any annexes for the publication of the notification of voting rights in writing to the e-mail address provided below.
We will keep you up to date!
To receive our publications automatically, simply register for the news distribution list.
Governance (IR)
Corporate Governance
Governance at Formycon
Formycon is committed to the principles of good corporate governance in respect of responsible, transparent and long-term successful management and control of the company. We follow the recommendations and principles of the German Corporate Governance Code.
Documents
Publications
Financial Reports
Annual, Half-Year and
Quarterly Reports
Key figures and information on the
company's development
Financial reports of recent years
Supervisory Board
Supervisory Board
Strategic advice with a broad perspective
The Supervisory Board of Formycon has many years of entrepreneurial experience.

From left to right: Colin Bond (Deputy Chairman of the Supervisory Board), Nicholas Haggar (Member of the Supervisory Board), Wolfgang Essler (Chairman of the Supervisory Board),
Klaus Röhrig (Member of the Supervisory Board), Dr. Bodo Coldewey (Member of the Supervisory Board).
Wolfgang Essler
Chairman of the Supervisory Board
Deputy Chairman of the Nomination and Remuneration Committee
Member since July 25, 2023
Elected until the end of the Annual General Meeting 2027
Wolfgang Essler, who was born on October 20, 1972 in Munich, Germany, studied business administration at the University of Augsburg from 1992 until 1998. He began his professional career in the consulting firms O&R Corporate Finance GmbH (1998-2008) and Duff & Phelps GmbH (2008-2010) with a focus on corporate finance and transactions, followed as a board member at the investment company balandis real estate ag and predecessor companies (2010-2023). Since 2021, he is chief representative (Generalbevollmächtigter) of ATHOS and managing director of Santo Holding (Deutschland) GmbH, i.e. of one of our Major Shareholder.
Alongside his office as a member of the Supervisory Board, Wolfgang Essler is, or was within the last five years, a member of the administrative, management or supervisory bodies of and/or a partner in the following companies and partnerships outside Formycon:
Currently:
- Vanguard AG, deputy chairman of the supervisory board;
- Mega Pharma Holding Uruguay S.A., Montevideo, Uruguay, member of the non-executive board of directors;
- WERK Immobilien GmbH, managing director;
- fidelius Investment GmbH, managing director;
- Santo Holding (Deutschland) GmbH, managing director;
- Terra Quantum AG, St. Gallen, Switzerland, member of the board of directors; and
- balandis real estate GmbH i.L., managing director and liquidator.
Previously: None
Other than listed above, Wolfgang Essler is not, and was not within the last five years, a member of any administrative, management or supervisory body of and/or a partner in any other company or partnership outside Formycon.
Colin Bond
Deputy Chairman of the Supervisory Board
Chairman of the Audit Committee
Member of the Nomination and Remuneration Committee
Member since October 1, 2024
Elected until the end of the Annual General Meeting 2028
Colin Michael Bond who was born on January 26, 1960 in Upminster, United Kingdom, holds a Bachelor of Science degree in pharmacy and a master’s degree in business administration from London Business School. During his early career, Colin Michael Bond worked as auditor and management consultant for PricewaterhouseCoopers, Arthur Andersen and Procter & Gamble. From 2010 to 2016, he served as Chief Financial Officer (CFO) of Evotec SE, which is listed on the Frankfurt Stock Exchange (Prime Standard) as well as NASDAQ. From 2016 to 2022, Colin Michael Bond served as Chief Financial Officer (CFO) of Vifor Pharma Management AG. From 2022 to June 2024, he served as Chief Financial Officer (CFO) of Sandoz AG. Colin Michael Bond is a Fellow of the Institute of Chartered Accountants in England and Wales, and a member of the Royal Pharmaceutical Society of Great Britain.
Alongside his office as a member of the Supervisory Board, Colin Michael Bond is, or was within the last five years, a member of the administrative, management or supervisory bodies of and/or a partner in the following companies and partnerships:
Currently:
- BioPharma Credit Plc, Leeds, United Kingdom, member of the board of directors.
- Agomab Therapeutics NV, Antwerp, Belgium, member of the board of directors
- Oxford Biomedica PLC, Oxford, United Kingdom, member of the board of directors
- Faron Pharmaceuticals Ltd., Turku, Finland, member of the board of directors
- Medichem S.A., Barcelona, Spain, member of the board of directors
Previously:
- Sandoz AG, Basel, Switzerland, member of the board of directors (from 2022 to 2024);
- Siegfried Holding AG, Zofingen, Switzerland, member of the board of directors (from 2013 to 2023); and
- Vifor Pharma Management AG, Glattbrugg, Switzerland, member of the board of directors (from 2016 to 2022).
Other than listed above, Colin Michael Bond is not, and was not within the last five years, a member of any administrative, management or supervisory body of and/or a partner in any other company or partnership.
Nicholas Haggar
Member of the Supervisory Board
Member of the Audit Committee
Chairman of the Nomination and Remuneration Committee
Member since June 12, 2024
Elected until the end of the Annual General Meeting 2028
Nicholas Haggar who was born on April 25, 1965 in Norwich, United Kingdom, has over thirty years of experience in building pharmaceutical businesses. With an Master of Business Administration from Cranfield School of Management and a Bachelors Degree in Industrial and Manufacturing Systems Engineering, Nicholas Haggar began his professional career in pharmaceutical technical operations before moving into commercial and enterprise leadership. Nicholas serves on the Zentiva board since 2023, having been Chief Executive Officer of Zentiva following its carve-out from Sanofi in 2018. Nicholas Haggar has been active in the biosimilar space for the last 15 years. Currently, he serves as Chief Executive of HealthQube Ltd., a UK-based investment, analytics and consultancy platform.
Alongside his office as a member of the Supervisory Board, Nicholas Haggar is, or was within the last five years, a member of the administrative, management or supervisory bodies of and/or a partner in the following companies and partnerships outside Formycon:
Currently:
- Zentiva K.S. International, Prague, Czech Republic, non-executive director;
- Biocon Limited, Bangalore, India, independent director;
- Healthqube Ltd, Chalfont St. Giles, United Kingdom, chief executive officer; and
- Polpharma Generic BV, non-executive chairman.
Previously:
- Zentiva K.S., Prague, Czech Republic, chief executive officer (from 2019 to 2023).
Other than listed above, Nicholas Haggar is not, and was not within the last five years, a member of any administrative, management or supervisory body of and/or a partner in any other company or partnership outside Formycon.
Klaus Röhrig
Member of the Supervisory Board
Member since December 10, 2020
Elected until the end of the Annual General Meeting 2029
Klaus Röhrig, who was born on July 21, 1977 in Vienna, Austria, studied business administration at the Vienna University of Economics and Business. He holds a Master of Economics and Business Administration from Vienna University of Economics and Business Administration. In 2000, he started his career at Credit Suisse First Boston in London, United Kingdom, focusing on corporate finance and M&A for technology companies. From 2002 to 2006, he worked for Mercury Capital GmbH in Vienna, Austria. In 2006, he joined Elliott Associates in London, United Kingdom, where he worked until 2012 and was responsible for the funds’ investments in the German speaking countries. Since 2015, he is founding partner and Co-Chief Investment Officer of Active Ownership Capital S.à r.l., an independent, partner-managed investment firm operating primarily in Continental Europe and Scandinavia.
Alongside his office as a member of the Supervisory Board, Klaus Röhrig is, or was within the last five years, a member of the administrative, management or supervisory bodies of and/or a partner in the following companies and partnerships outside Formycon:
Currently:
- Agfa-Gevaert N.V., Belgium, member of board of directors (non-executive director);
- Fagron NV, Belgium, member of board of directors (non-executive director);
- R3 Capital GmbH, Vienna, Austria, managing director;
- R3ND Immobilien GmbH, Vienna, Austria, managing director;
- Mercury Capital GmbH, Vienna, Austria, managing director;
- MAM Baby AG, Wollerau, Switzerland, member of the board of directors;
- Active Ownership Research Austria GmbH, Vienna, Austria, managing director;
- Active Ownership Corporation S.à. r.l., Grevenmacher, Luxembourg, member of the management board;
- Active Ownership Capital S.à. r.l., Grevenmacher, Luxembourg, member of the management board;
- White Elephant Holdco S.à. r.l., Grevenmacher, Luxembourg, member of the management board;
- White Elephant S.à. r.l., Grevenmacher, Luxembourg,, member of the management board;
- AOC Technology SAS, Grevenmacher, Luxembourg, member of the management board;
- AOC Value SAS, Grevenmacher, Luxembourg, member of the management board;
- H2APEX Management S.à. r.l., Grevenmacher, Luxembourg, member of the management board;
- AOC Health Holdco S.à. r.l., Grevenmacher, Luxembourg, member of the management board;
- AO Gaming S.à. r.l., Grevenmacher, Luxembourg, member of the management board;
- AOC Cloud S.à. r.l., Grevenmacher, Luxembourg, member of the management board;
- AOC Fox S.à r.l., Grevenmacher, Luxembourg, member of the management board;
- AOC Alpha S.à r.l., Grevenmacher, Luxembourg, member of the management board;
- Aonic Holdco S.à. r.l., Grevenmacher, Luxembourg, member of the management board;
- Aonic Holdco 2 S.à. r.l., Grevenmacher, Luxembourg, member of the management board;
- AO MLP S.à. r.l., Grevenmacher, Luxembourg, member of the management board; and
- AOC Pharma S.à. r.l., Grevenmacher, Luxembourg, member of the management board.
- EOF Master Holdco S.à r.l., Grevenmacher, Luxembourg, member of the management board;
Previously: none
Other than listed above, Klaus Röhrig is not, and was not within the last five years, a member of any administrative, management or supervisory body of and/or a partner in any other company or partnership outside Formycon.
Dr. Bodo Coldewey
Member of the Supervisory Board
Member of the Audit Committee
Member since June 12, 2024
Elected until the end of the Annual General Meeting 2027
Dr. Bodo Coldewey, who was born on December 16, 1971 in Oldenburg, Germany, completed his studies in business engineering at the Technical University of Kaiserslautern and his doctorate at the Leipzig Graduate School of Management. In 1997, he began his professional career as consultant at KPMG Deutschland, where he worked until June 2000. In July 2000, he joined 3i Group plc as investment manager, where he worked until September 2002. From October 2002 to December 2011, Dr. Bodo Coldewey served as director of Oldenburgische Landesbank AG. Since 2012, he is managing director of the family office WEGA Invest GmbH.
Alongside his office as a member of the Supervisory Board, Dr. Bodo Coldewey is, or was within the last five years, a member of the administrative, management or supervisory bodies of and/or a partner in the following companies and partnerships outside Formycon:
Currently:
- WEGA Support GmbH, managing director;
- WEGA Invest GmbH, managing director;
- WEGA Beteiligungsverwaltungsgesellschaft mbH, managing director;
- Dozena GmbH, managing director;
- PW Garrel Verwaltungs GmbH, managing director;
- BW Garrel Verwaltungs GmbH, managing director; and
- WEGA EQUITY AUDA Beteiligungs-GmbH i.L., managing director and liquidator.
Previously: None
Other than listed above, Dr. Bodo Coldewey is not, and was not within the last five years, a member of any administrative, management or supervisory body of and/or a partner in any other company or partnership outside Formycon.
Financial Calendar
Financial Calendar
Publications,
Webcasts and Events
Formycon believes in open dialogue with its investors and with the capital markets, as an integral part of its corporate philosophy. In addition to the publication dates for our financial results and the Annual General Meeting, this section provides an overview of the investor conferences that representatives of the Management Board will be attending in the coming weeks.
August 13, 2025
Publication of Half-Year Report 2025
Planegg-Martinsried
August 28, 2025
HIT – Hamburg Investors' Days
Hamburg
Further Information
September 08 – 10, 2025
H.C. Wainwright 27th Annual Global Investment Conference
New York City
September 22 – 24, 2025
Berenberg & Goldman Sachs German Corporate Conference
Munich
September 24 – 26, 2025
H.C. Wainwright – Biotech "On Tap" 2025
Munich
November 13, 2025
Publication of Nine-month Figures 2025
Planegg-Martinsried
November 24 – 26, 2025
Deutsche Börse Equity Forum
Frankfurt
June 18, 2025
Annual General Meeting
Haus der Bayerischen Wirtschaft, Munich
June 13, 2025 · 10 a.m.
mwb research roundtable
virtual
Register
June 12, 2025
Warburg Highlights Conference
Hamburg
June 11, 2025
UBS Life Science Conference 2025
London
May 21 – 22, 2025
Berenberg European Conference
New York
May 12 – 14, 2025
Equity Forum Spring Conference
Frankfurt
Further Information
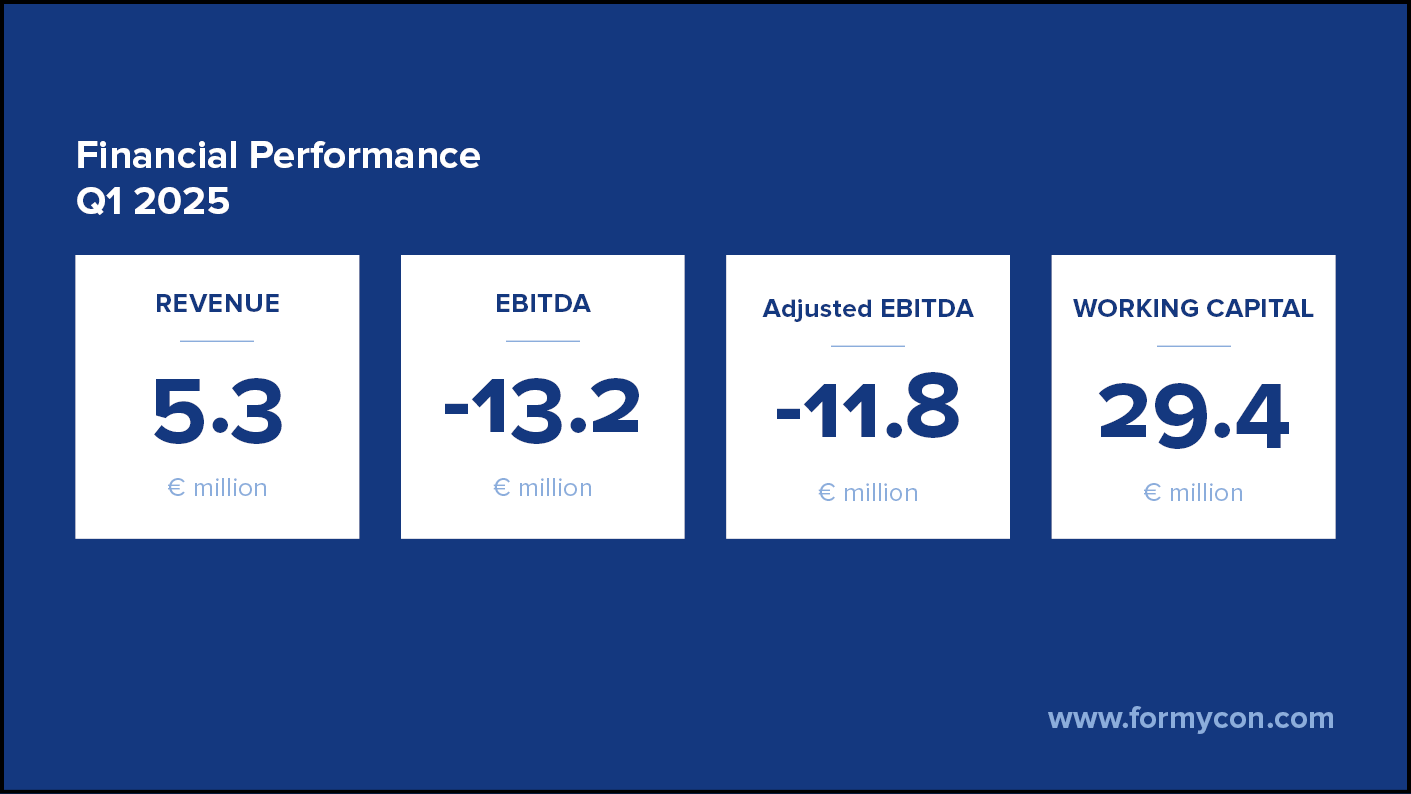
May 12, 2025
Publication of the Result for the first Quarter of 2025
Planegg-Martinsried
April 8, 2025 · 10:00 – 10:30 CEST
mwb research German Select Conference
virtual
Replay
April 3, 2025
Metzler Small Cap Days 2025
Frankfurt
Further Information
March 27, 2025
Publication of the Annual Report 2024
Planegg-Martinsried
February 13, 2025
ODDO BHF Small Cap Conference
Frankfurt
January 21, 2025
UniCredit & Kepler Cheuvreux German Corporate Conference
Frankfurt
Further Information
January 13 – 16, 2025
J.P. Morgan 43rd Annual Healthcare Conference
San Francisco
Further Information
December 12, 2024 at 11am (CET)
MWB Research Roundtable
virtual / held in german
Replay
December 02, 2024
H.C. Wainwright virtual Fireside Chat
virtual
November 28, 2024
Publication of nine-month figures
Planegg-Martinsried
Further Information
November 25 – 27, 2024
Deutsche Börse Eigenkapitalforum
Frankfurt
Further Information
November 19 – 21, 2024
Jefferies London Healthcare Conference
London
Further information
November 05, 2024
Berenberg virtual Fireside Chat
virtual
September 23 – 25, 2024
Berenberg & Goldman Sachs German Corporate Conference
Munich
September 19, 2024
Pareto Securities' 15th. Annual Healthcare Conference
Stockholm
September 09 – 11, 2024
H.C. Wainwright 26th Annual Investment Conference
New York City
August 21 – 22, 2024
HIT - Hamburg Investor Days
Hamburg
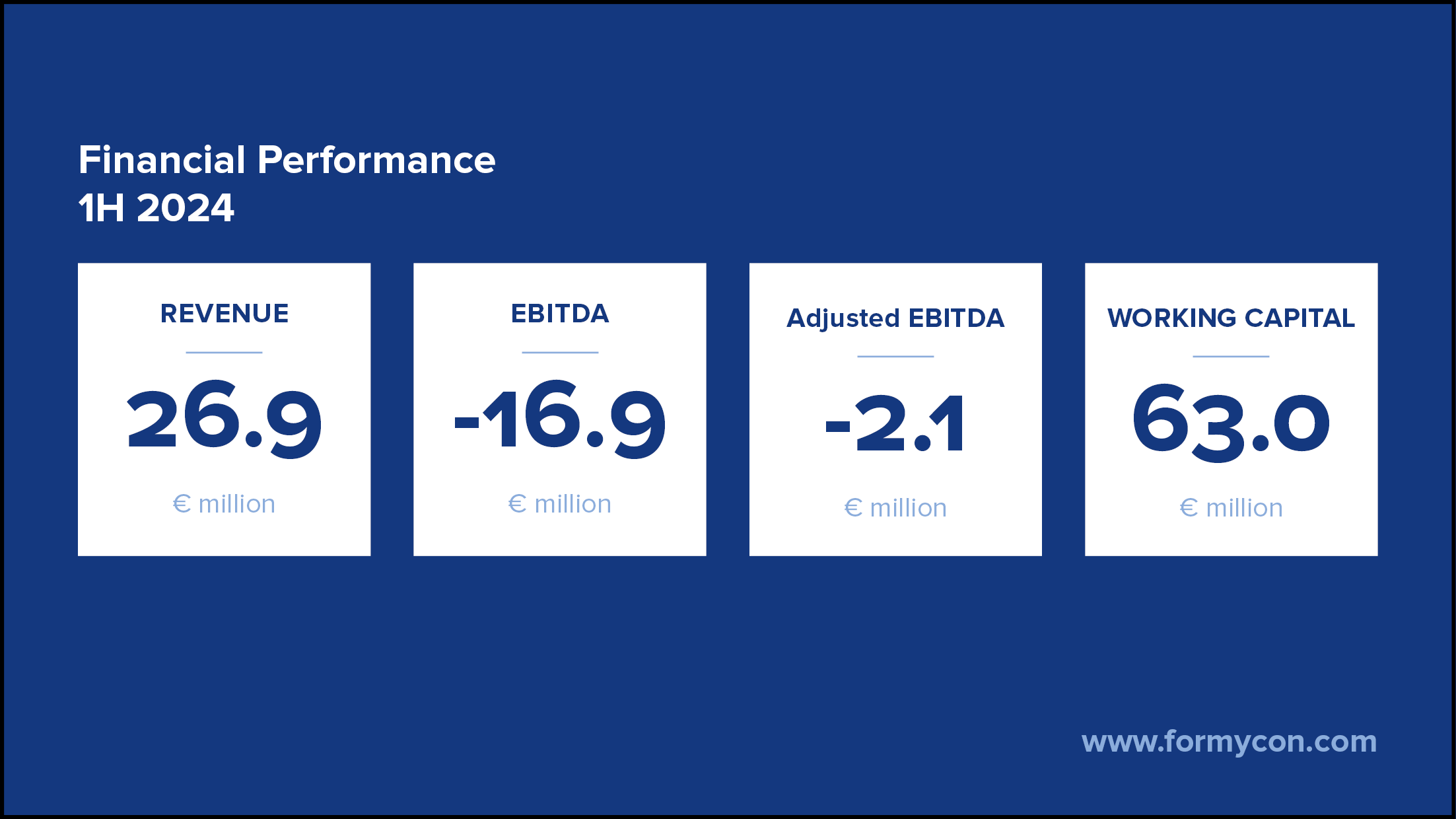
August 13, 2024
Publication of Half-Year Report
Planegg-Martinsried
Further information
June 12, 2024
Annual General Meeting
Haus der Bayerischen Wirtschaft, Munich
Further information
June 06 – 07, 2024
Warburg Highlights
Hamburg
June 05 – 06, 2024
Jefferies Global Healthcare Conference
New York City
June 03, 2024
MWB Research Roundtable
virtual
Webcast
May 21 – 23, 2024
10th Berenberg European Conference
New York City
May 15 – 17, 2024
Hauck Aufhäuser Stockpicker Summit
Kitzbühel
May 13 – 15, 2024
Equity Forum Spring Conference
Frankfurt
May 08, 2024
Publication of the result for the first quarter of 2024
Planegg-Martinsried
Further information
April 30, 2024
Jefferies Fireside Chat
virtual
April 25, 2024
Publication of the annual report 2023
Planegg-Martinsried
Further information
April 22 – 24, 2024
Metzler Small Cap Days
Frankfurt
March 19 – 21, 2024
Jefferies Pan-European Mid-Cap Conference
London
March 12 – 14, 2024
J.P. Morgan Annual Pan-European Small/Mid-Cap CEO Conference
London
March 04 – 07, 2024
Berenberg EU Opportunities Conference 2024
London
January 17, 2024
UniCredit & Kepler Cheuvreux German Corporate Conference
Frankfurt
January 15 – 16, 2024
Oddo BHF Forum
virtual
Januar 09 – 12, 2024
J.P. Morgan Healthcare Conference
San Francisco
2025
Ad-Hoc Releases
Ad-Hoc Releases
Disclosure of inside information according to Article 17 of the Regulation (EU) No 596/2014
Formycon successfully places an EUR 70 million senior unsecured floating rate bond
The management board of Formycon AG today successfully placed a senior unsecured floating rate bond in a total volume of EUR 70 million.
June 27, 2025
Early end of the offer period for the bond due to high demand
The announced corporate bond of Formycon AG has met with great interest, particularly among institutional investors. Due to the strong demand, the offer period of the public offer of the Bond will be shortened.
June 24, 2025
Formycon plans to issue a public senior unsecured floating rate bond with target volume of EUR 50 million to support further company growth
The Management Board of Formycon AG today resolved, with the approval of the Supervisory Board, to examine the possibility of issuing a public senior unsecured, floating rate corporate bond with a target volume of EUR 50 million to support its ongoing growth strategy.
June 17, 2025
Formycon announces decision on Phase III trial with FYB206 and provides update on potential need to adjust the valuation of FYB202 and FYB201
Due to the positive feedback from the FDA, the development of FYB206 can continue without a Phase III clinical trial. Increasing and emerging price discounts in the USA are likely to require impairments of FYB201 and FYB202.
February 17, 2025
Aflibercept-Biosimilar FYB203 / AHZANTIVE®/ Baiama® receives positive CHMP Opinion from EMA
Formycon announces that the European Medicines Agency published today that the Committee for Medicinal Products for Human Use issued a positive opinion for market approval for FYB203 / AHZANTIVE®/ Baiama®, a biosimilar candidate to Eylea® (Aflibercept).
November 15, 2024
FDA grants approval for Stelara® Biosimilar FYB202/Otulfi™ (ustekinumab-aauz)
Formycon AG announces that the U.S. Food and Drug Administration (FDA) today approved FYB202/OtulfiTM (ustekinumab-aauz), a biosimilar to Stelara®.
September 27, 2024
Formycon AG increases its outlook for the 2024 fiscal year
Formycon AG has today decided, based on the preliminary half-year figures, to raise its guidance for the 2024 fiscal year. This affects the key figures adjusted EBITDA and working capital.
August 6, 2024
Stelara® Biosimilar Candidate FYB202 (Ustekinumab) receives positive CHMP opinion from EMA
Formycon AG announces that the Committee for Medicinal Products for Human Use of the European Medicines Agencytoday issued a positive opinion for FYB202, a biosimilar candidate to Stelara® (Ustekinumab).
July 26, 2024
FDA grants approval for Eylea®1) biosimilar FYB203/AHZANTIVE®2) (aflibercept-mrbb)
Based on preliminary and unaudited figures for the 2023 financial year, Formycon AG (ISIN: DE000A1EWVY8 / WKN: A1EWVY) ("Company") expects EBITDA to be around EUR 1.5 million (guidance: EUR -5 million to EUR -15 million)
June 28, 2024
Formycon AG publishes preliminary figures for the 2023 financial year and guidance for the 2024 financial year
Based on preliminary and unaudited figures for the 2023 financial year, Formycon AG (ISIN: DE000A1EWVY8 / WKN: A1EWVY) ("Company") expects EBITDA to be around EUR 1.5 million (guidance: EUR -5 million to EUR -15 million)
April 12, 2024
Formycon resolves on cash capital increase of EUR 82.84 million – all new shares were subscribed by Gedeon Richter as new strategic investor
Today, the management board of Formycon AG, with the consent of the Company's supervisory board, resolved to increase the Company's share capital against cash contributions by issuing new no par-value bearer shares of the Company, partially utilizing the authorized capital and excluding the shareholders' subscription rights.
January 29, 2024
Formycon AG announces result of private placement and sets placement price for the new shares from the capital increase
Munich – On the basis of the bookbuilding procedure carried out as part of the private placement, the Management Board, with the approval of the Supervisory Board, set a placement price of EUR 77.00 per new share, resulting in gross proceeds from the offering of EUR 70,070,000.00 before commissions and costs. The New Shares correspond to approximately 6.02% of the Company's currently issued share capital.
February 2, 2023
Formycon AG upsizes capital increase from authorized capital following strong demand
Munich – Formycon AG (ISIN: DE000A1EWVY8 / WKN: A1EWVY) ("Formycon” or “Company") announces that, following strong demand, the Company has decided to upsize their previously announced capital increase from authorized capital from approx. 5% to in total approx. 6% of the outstanding share capital.
February 1, 2023
Formycon AG plans capital increase of approx. 5% of the share capital by way of an accelerated bookbuilding procedure to finance further growth
Munich – Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) intends to issue approx. 5% of the issued share capital of the Company, representing approx. 750,000 new shares (the "New Shares").
February 1, 2023
Formycon AG announces global license agreement with Fresenius Kabi for FYB202, a biosimilar candidate to Stelara®1 (ustekinumab)
Munich – Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY), as the exclusive owner of the global commercial rights to FYB202, a biosimilar candidate to Stelara® (ustekinumab), today announced the conclusion of a global license agreement with Fresenius Kabi AG (“Fresenius Kabi”).
February 1, 2023
Formycon announces binding Term Sheet between Klinge Biopharma and Coherus BioSciences for the exclusive commercialization of FYB203, a biosimilar candidate to Eylea®1, in the United States
Munich – Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) announces that Klinge Biopharma GmbH, the exclusive owner of the global commercialization rights of FYB203, Formycon’s biosimilar candidate to Eylea® (aflibercept), has entered into a binding term sheet with Coherus BioSciences, Inc. (“Coherus”) for the exclusive commercialization of FYB203 in the United States (U.S.).
January 9, 2023
Formycon announces EU-Approval of FYB201/Ranivisio®1 a biosimilar to Lucentis®2
Munich – Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) announces that the European Comission (“EC”) today has granted marketing authorization (“MA”) for Ranivisio®, a biosimilar to Lucentis® (ranibizumab-injection) for medical use in the European Union (“EU”).
August 26, 2022
Formycon announces FDA approval of FYB201/CIMERLITM1 (ranibizumab-eqrn) as a Biosimilar interchangeable with Lucentis®2
Munich – Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) announces that the U.S. Food and Drug Administration (“FDA”) has approved CIMERLI™ (ranibizumab-eqrn), a biosimilar product interchangeable with Lucentis® (ranibizumab injection).
FYB201 was developed by Bioeq AG, a Joint...
August 3, 2022
FYB201, Formycon’s biosimilar for Lucentis® (ranibizumab), receives CHMP recommendation from EMA
Munich - Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) and its licensing partner Bioeq AG ("Bioeq") announce that the Committee for Medicinal Products for Human Use ("CHMP") of the European Medicines Agency ("EMA") today issued a positive opinion for FYB201, a biosimilar to...
June 24, 2022
Formycon announces changes to its Executive Board
Munich – Today and effective July 1, 2022, the Supervisory Board of Formycon AG (ISIN: DE000A1EWVY8 / WKN: A1EWVY) ("Formycon") appointed Dr. Stefan Glombitza, who has led Formycon's operational development activities as Chief Operating Officer (COO) since 2016, as Chief Executive Officer...
May 19, 2022
FYB201, Formycon’s Biosimilar for Lucentis®¹, achieves Marketing Authorization in United Kingdom
Munich – Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) and its license partner Bioeq AG (“Bioeq”) announce, that today the Medicines and Healthcare products Regulatory Agency (MHRA) has granted marketing authorization (MA) in the United Kingdom (“UK”) for FYB201, a biosimilar to...
May 17, 2022
Formycon and ATHOS KG merge development activities through the acquisition of biosimilar assets in a long-term strategic partnership
Munich - Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) and SCG Cell Therapy Pte Ltd ("SCG") today announced that they have entered into a collaboration and license agreement for the development and commercialization of Formycon's proprietary COVID-19 drug FYB207. Under the terms of the agreement,...
March 29, 2022
Formycon Concludes Collaboration and License Agreement with SCG Cell Therapy for its COVID-19 Drug FYB207 for the Asia Pacific Region
Munich - Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) and SCG Cell Therapy Pte Ltd ("SCG") today announced that they have entered into a collaboration and license agreement for the development and commercialization of Formycon's proprietary COVID-19 drug FYB207. Under the terms of the agreement,...
October 18, 2021
Formycon and Bioeq announce File Acceptance for FYB201, a biosimilar candidate to Lucentis® (ranibizumab) by the U.S. Food and Drug Administration (FDA)
Munich - Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) and its license partner Bioeq AG (“Bioeq”) announce that the U.S. Food and Drug Administration (FDA) accepted the biologics license application (BLA) for FYB201, a biosimilar candidate to Lucentis®1, for review and assigned a target action date for the application for August 2022.
October 1, 2021
Formycon and Bioeq announce submission of the biologics license application (BLA) for FYB201, a biosimilar candidate to Lucentis(R)1 (ranibizumab) to the U.S. Food and Drug Administration (FDA)
Munich - Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) and its license partner Bioeq AG ("Bioeq") announce that the biologics license application (BLA) for FYB201, Formycon's biosimilar candidate to Lucentis(R), has been recently submitted to the U.S. Food and Drug Administration...
August 5, 2021
Formycon receives Euro 12.7 million grant for further development of COVID-19 drug FYB207 as part of the Bavarian Therapy Strategy to combat the COVID-19 pandemic
Munich - Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) announced today that it has received the final notification for a grant from the Bavarian Ministry of Economic Affairs, Regional Development and Energy in the amount of Euro 12.7 million to support the further development of the COVID-19 drug...
July 12, 2021
Formycon and Bioeq announce submission of the marketing authorization application for FYB201, a biosimilar candidate to Lucentis(R)1 (ranibizumab) to the European Medicines Agency (EMA)
Munich - Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) and its license partner Bioeq AG announce that the marketing authorization application (MAA) for FYB201, Formycon's biosimilar candidate to Lucentis(R) (ranibizumab), has been submitted to the European Medicines Agency...
June 29, 2021
Formycon announces conclusion of exclusive commercialization agreement between Bioeq AG and Teva Pharmaceutical Industries Ltd. for FYB201 in Europe, Canada, Israel and New Zealand
Munich - Bioeq AG ("Bioeq"), the exclusive owner of the global commercialization rights of Formycon's biosimilar candidate to Lucentis(R)1 (ranibizumab), informed Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY), that an exclusive strategic partnership for commercialization of FYB201 in...
June 28, 2021
Formycon informs about the modified BLA-Submission Strategy for its Lucentis(R)* Biosimilar-Candidate FYB201
Munich - Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) and its licensing partner Bioeq AG ("Bioeq") announced today that the Biologics License Application (BLA) resubmission strategy for FYB201 has been adjusted.
The approval for FYB201 will be requested directly for a large commercial...
November 5, 2020
Formycon places Cash Capital Increase of 25.75 Million Euros with Strategic Investor
Munich - The Management Board of Formycon AG (ISIN: DE000A1EWVY8/ WKN: A1EWVY) resolved yesterday, with the approval of the Supervisory Board, to increase the company's capital against cash contributions by partially utilizing the approved capital. The Company's share capital of currently EUR...
October 12, 2020
Formycon informs about the current Status of the BLA Review Process of the Lucentis®Biosimilar Candidate FYB201
Formycon AG and its licensing partner Bioeq announce today, that the U.S. Food and Drug Administration (FDA) has requested additional data as part of the review process of the BLA for the Lucentis® biosimilar candidate FYB201, submitted by Bioeq in December 2019.
February 4, 2020
Bioeq IP AG Exclusively Licenses US Marketing Rights For FYB201 To Coherus BioSciences, Inc.
Bioeq IP AG, the exclusive owner of the global commercialization rights of Formycon’s FYB201 has signed a license and development agreement with Coherus BioSciences, Inc. The US Biosimilar-specialist, headquartered in California, will exclusively market and distribute Formycon's biosimilar candidate to Lucentis® (FYB201) in the United States of America (US).
November 6, 2019
Formycon Reports Further Course of Capital Measure
With the agreement dated June 26, 2019, Wendeln & Cie. KG, an asset management company of Mr. Peter Wendeln (anchor shareholder and long-standing member of the Supervisory Board of Formycon AG), acquired all rights from the certificate of subscription as well as the 577,397 shares in Formycon AG from the cash capital increase.
June 26, 2019
Formycon Reports Latest Status of Capital Increase
As of today, totaling EUR 5,000,000.00, have been paid to the company’s capital increase account. The signatory's debt contribution amounting to EUR 12,264,170.30 is therefore still outstanding. The shares have so far not been delivered due to the as yet unsettled payment.
June 3, 2019
Formycon Places Cash Capital Increase of EUR 17.3 Million with Institutional Investor
Strategic investor M&H Equity AG subscribes for a cash capital increase of € 17,264,170.30 million in the context of a private placement with an issue price of 29.90 EUR per share. The funds acquired from the capital increase will be used primarily to develop the company’s own biosimilar products, particularly FYB205.
March 22, 2019
Biosimilar Candidate FYB201 Shows Efficacy Comparable to the Reference Product in Phase III Study
According to an interim result, the primary endpoint has been achieved in the COLUMBUS-AMD phase III trial, which is intended to demonstrate the efficacy, safety and immunogenicity of FYB201 and the reference medicinal product Lucentis® in patients with neovascular age-related macular degeneration (nAMD).
May 2, 2018
Formycon and Aristo Pharma are Founding a Joint Venture for the Development of FYB202
Formycon and Aristo Pharma GmbH, a company of the Struengmann-Group, have formed a joint company for the continued development of FYB202, Formycon's biosimilar candidate for Stelara® (ustekinumab).
December 22, 2017
Formycon signs term sheet for development of FYB202 and executes capital increase
Formycon plans to enter into a co-investment arrangement for its biosimilar candidate FYB202 and aims for a participation of up to 30% in worldwide marketing proceeds.
July 24, 2017