Pipeline
Building a biosimilar product portfolio for the long term
Formycon covers the entire range of biosimilar medicines development, from technical-pharmaceutical development to clinical trials, all the way through to preparation and submission of dossiers for regulatory approval by international authorities.
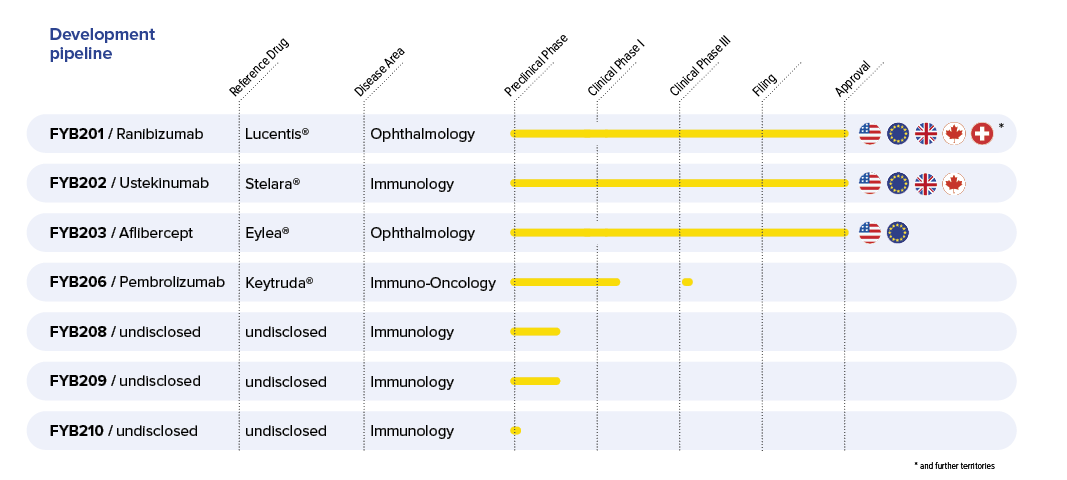
The company currently has several biosimilar product candidates in advanced development – FYB201, FYB202, FYB203, FYB206 as well as the undisclosed candidates FYB208, FYB209 and FYB210 – with each of these positioned to potentially compete against existing blockbuster biopharmaceuticals, each with sales in the billions of dollars. The market potential for our product candidates is thus substantial.
Formycon holds 50% of the rights in FYB201, a biosimilar candidate for Lucentis®. Formycon holds 100% of the rights in FYB202, a biosimilar candidate for Stelara®, in FYB206, a biosimilar candidate for Keytruda® as well as FYB208, FYB209 and FYB210 as yet unpublished biosimilar candidates. FYB203, a biosimilar candidate for Eylea® is in a licensing partnership with Klinge Biopharma GmbH, a Santo Holding (Deutschland) GmbH company.
Formycon regularly publishes updated information regarding the advances in its biosimilar development pipeline, which is expected to be further expanded in the coming years.
¹ Lucentis® is a registered trademark of Genentech, Inc.
² Stelara® is a registered trademark of Johnson & Johnson
³ Eylea® is a registered trademark of Regeneron Pharmaceuticals, Inc.
4 Keytruda® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ/USA.