Social
Diversity, openness and social justice
We stand up - for diversity, openness and social justice, not only for our own employees, but along the entire value chain.

Employees
Diversity and Equality
We are #TeamFormycon! Formycon currently employs more than 240 committed employees from over 30 nations, of which around 60% are women. More than 80% of our colleagues work in research and development.

We recognize the power of diversity and promote it wherever we can. We are committed to a world in which every person – regardless of origin, gender, religion or other characteristics – enjoys the same opportunities and rights. Especially in today’s world, we stand by these values, which are essential for us, our company and our future. All our employees are obliged to familiarize themselves with the General Equal Treatment Act (AGG) through appropriate training when they join the company. This training also includes the Formycon Diversity, Equity and Inclusion policy that prohibits discrimination or harassment of any kind in any of our employment practices, including recruitment, hiring, training, promotion or other matters.
We are proud of our organization, which has grown steadily over the years, and the values we live by, openness, tolerance, reliability, appreciation and mutual trust. Since 2022, Formycon is actively supporting the LGBTQIA+ community in the company by establishing special communication channels such as “FOR_MY_Queers_Community”, enabling an exchange on the company’s intranet. A separate LGBTQIA+ podcast series on topics such as diversity or international LGBTQIA+ rights not only provides information, but also sensitizes employees and promotes understanding and tolerance.
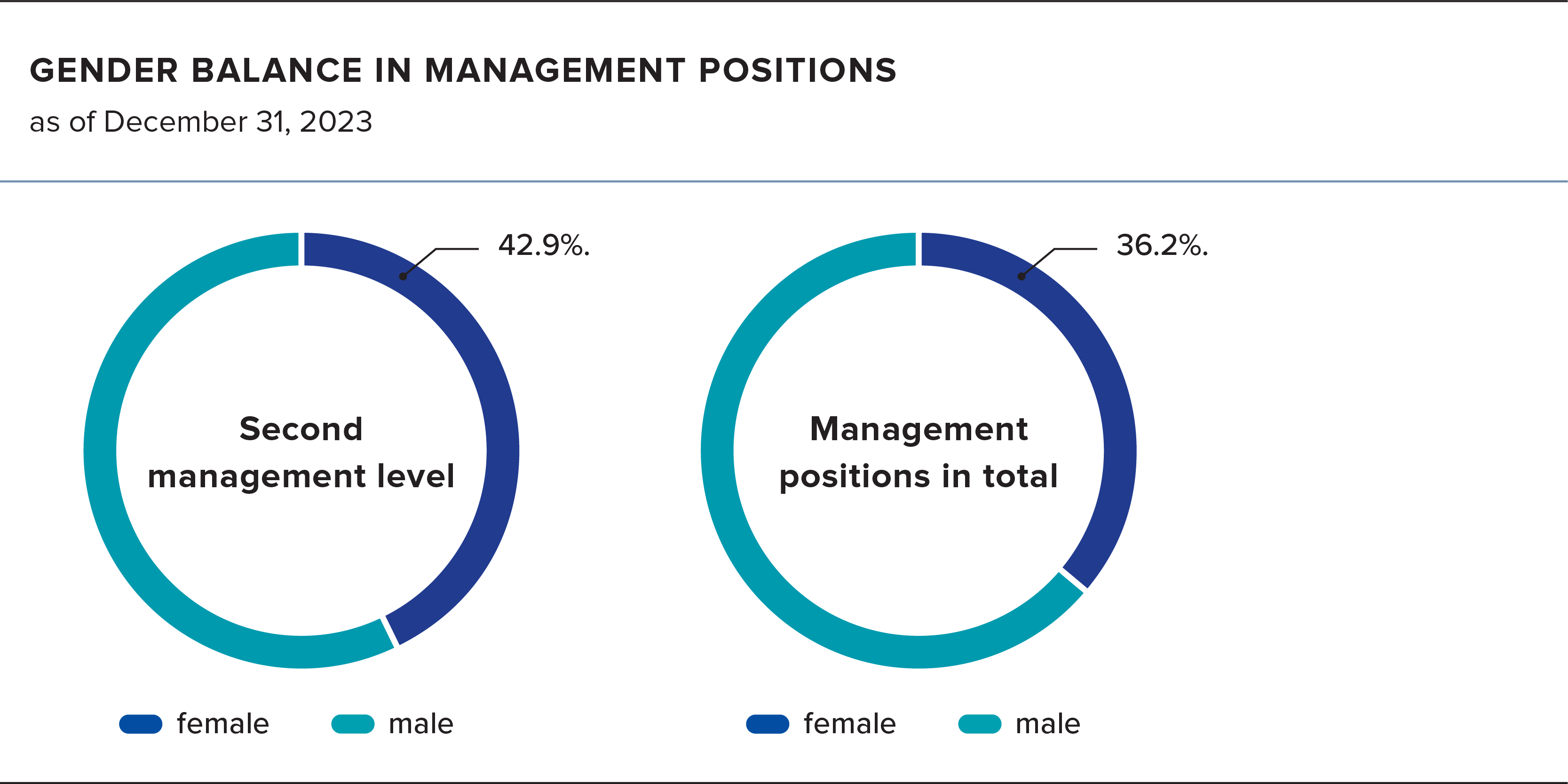
Formycon takes the issue of women’s leadership and growth very seriously and strives to provide the work environment to achieve this. The proportion of women at the second management level (Vice President, Senior Director, Director and Associate Director) was 42.9% as of December 31, 2023. At the highest management level (Board of Directors), the proportion was 25%. Altogether, the proportion of women at Formycon across all management positions is 36.2%
Occupational health and safety
Our employees are at the heart of our success, therefore work safety and the protection of employees are top priorities for Formycon. Operational processes can only run smoothly if health and safety protection is implemented in a practice-oriented way. Formycon holds the “Safe with System” seal of approval from the German employers’ liability insurance association for the raw materials and chemical industry (BGRCI). As part of the voluntary audit, both the occupational health and safety management system (AMS) and the effectiveness of the occupational health management system (BGM) must be examined, for the seal of approval to be awarded. In addition to the biological safety officer, the project manager in accordance with the German Genetic Engineering Act and the safety specialist, we have entrusted several experienced employees with special tasks in the field of occupational safety and protection. Supported by guidelines, training and regular medical check-ups, Formycon pursues the goal of minimizing the likelihood of accidents at work and at the same time ensuring the safety and well-being of our entire workforce.
Formycon attaches great importance to the health of its employees and offers, in addition to the mandatory examinations, flu vaccinations and consultations on ergonomic working as well as first aid courses, all provided by the company physician. Together with an external service provider, we regularly conduct anonymous surveys on psychological risk to identify any potentials for improvement. We also offer our employees access to an online mental health platform that provides professional psychological counseling online. In addition, measures that are intended to further improve the processes implemented to ensure health protection are identified and implemented on a continuous basis.
Training and skills development
The #Formycon Team is first-class, committed and operates at the cutting edge of medical research. A positive and productive atmosphere is essential for us, and we attach great importance to learning from each other and developing professionally.
To achieve this, Formycon offers individual opportunities for further education and training at a professional level for all employees. In addition to a Scientific and Clinical Career Path for Formycon scientific staff, a Managerial Career Path for employees of the Regulatory Affairs, Quality Management and Project Management departments has been implemented to promote personal career planning within the company. Formycon supports participation in seminars, congresses and lectures both in-house and with external partners. There are not only seminars on technical skills available, but our employees can also choose to attend training in areas such as languages, personal coaching, resilience and leadership. We also want to set an example for the next generation and plan to expand our trainee program in several specialist areas in addition to our existing apprenticeships in the field of IT.

Value chain
Responsibility in the value chain
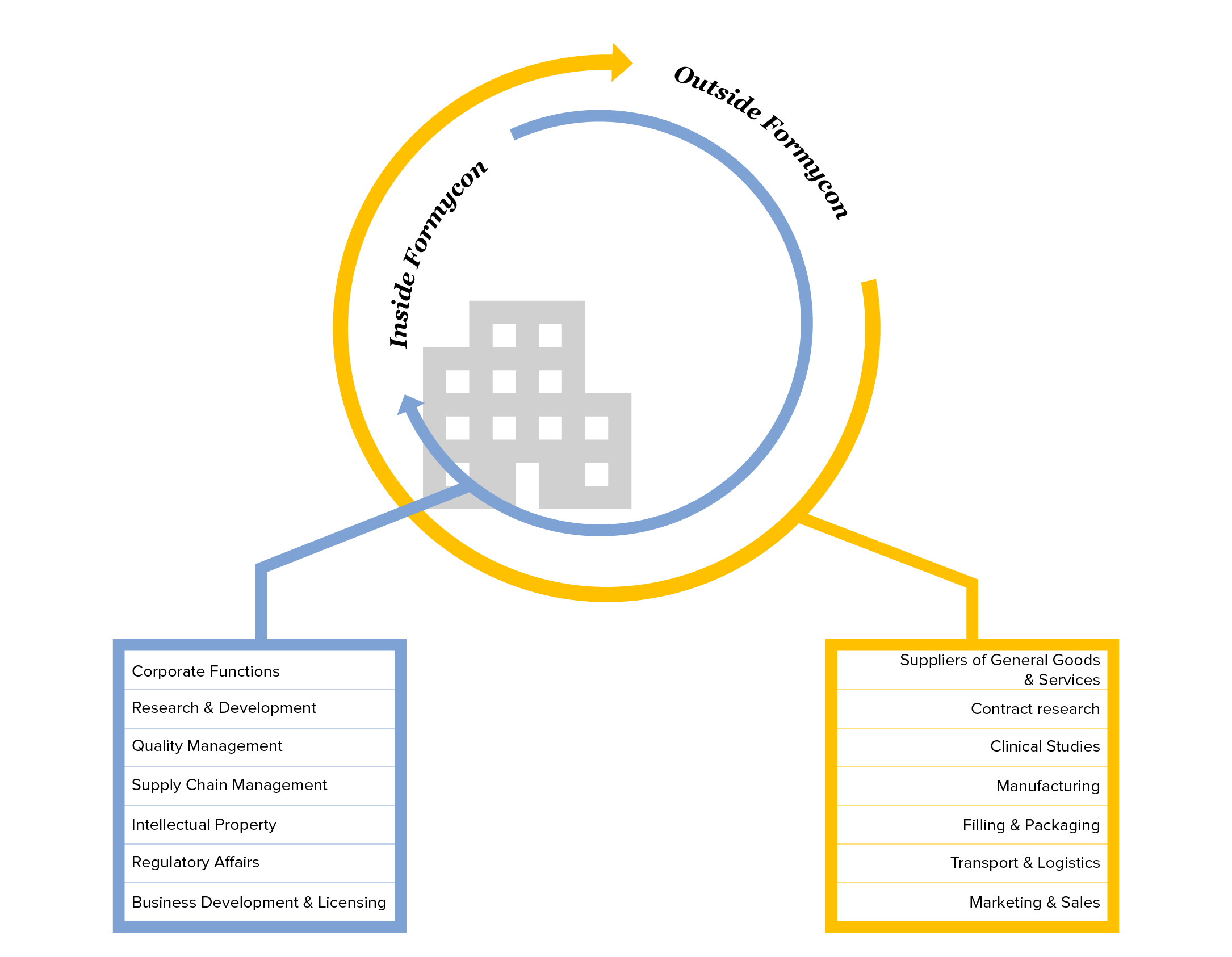
In addition to taking responsibility for our own employees, Formycon is committed to respecting human rights throughout the value chain. We want to ensure that all involved in the value chain are treated with dignity and respect, contributing thus to sustainable development and social justice. As a biosimilar developer, we do not have own manufacturing facilities nor do we source materials in large quantities. Therefore, we aim to work with strategic business partners to uphold human rights along the value chain. To this end, we have created our Supplier Code of Conduct, which explains our values to our partners and makes it clear that we expect them to act in accordance with the Code of Conduct. It is based on the 10 principles of the United Nations (UN) Global Compact, the UN Guiding Principles on Business and Human Rights, the OECD Guidelines for Multinational Enterprises and the conventions of the International Labour Organization (ILO). We will also increasingly include ESG criteria in the selection of our suppliers and business partners. In doing so, we seek dialogue and engagement with our partners to increase the transparency of the value chain and to ensure that human rights are respected.
Patients
We are driven by the aim to improve access to modern and effective therapies for patients who have so far been underserved. Our contribution is to develop biosimilars and market them at a lower cost than the original substances. In this way, Formycon not only helps patients worldwide, but also contributes to the sustainable financial relief of healthcare systems.
The safety and quality of our medicines for our patients is a top priority. The development of biosimilars for highly regulated markets requires high standards of safety, quality and efficacy of the drugs. The quality assurance requirements for the production processes related to medicinal products and active ingredients are defined by the European Commission in the Principles and Guidelines of Good Manufacturing Practice (GMP) for medicinal products for human use. Formycon’s laboratories are managed under these guidelines and recurringly inspected and audited by regulatory bodies such as the U.S. Food and Drug Administration (FDA).

„Taking responsibility together – not just for our projects but on a larger scale with regard to social and environmental issues – is an important step towards a more sustainable future. I’m glad that such high importance is placed on sustainability themes at #TeamFormycon.“
Qiyun Tang
Associate Manager Regulatory Affairs
The conduct of clinical trials concerning medicinal products for human use is strictly regulated by the Good Clinical Practice (GCP) regulation. The GCP Regulation is valid worldwide and serves to protect patients and the integrity and accuracy of the data and findings generated during such studies. We monitor the compliance with all relevant regulations and standards very closely, not only for us, but also for our business partners.
We ensure the high quality and safety of our products for patients now and in the future and are working to make them available globally. In addition, we conduct research on and develop more patient-friendly methods to administer our products.
Animal welfare
At Formycon, all our products must be safe, effective, and efficient and developed in full compliance with legal requirements. For the development of biosimilars, it is usually not legally required to test the drug substances on animals. However, in rare cases, we are mandated by law to conduct testing of our products on animals before clinical trials can take place on humans.
Formycon is aware that animal experiments raise a special responsibility. For this reason, we not only comply with all laws and regulations regarding animal testing, but we also enact strict internal rules to ensure that they are treated respectfully and ethically. These rules are based on the 3R principle – to completely avoid animal experiments (Replace), and to minimise the number of animals (Reduction) and their suffering (Refinement) in experiments as much as possible.
A careful evaluation takes place before each study, especially regarding the purpose of the study, the effects on the animals and the reasons why no results can be obtained otherwise. The study will only take place if animal experiments are prescribed by mandatory regulation or results cannot be obtained by an alternative method. In addition, all employees who participate must be properly trained and qualified to conduct animal testing. Of course, this also applies to our business partners and their employees.
