Biosimilars
Similar in quality, efficacy and safety
Biosimilars represent a new class of highly effective medicines at affordable prices
What are biosimilars?
Biosimilars are biologic drugs which are approved based upon their high similarity to existing “reference” biopharmaceuticals, specifically in terms of their quality, safety and therapeutic efficacy. Biosimilar medicines are submitted for regulatory approval in the world’s most stringently regulated markets, including the European Union, the United States, Canada, Japan and Australia, so that they may be brought to market upon their term of patent expiration of on the respective reference drug.
Biosimilar medicines represent a rapidly growing new area of pharmaceutical biotechnology, analytical science and clinical research. Their development requires a fundamentally different approach to that used for originator biopharmaceuticals, with analytical design playing a particularly important role. Biosimilars are produced under strictly controlled conditions, using state-of-the-art biotechnology to ensure the highest quality standards.
Comparison of biosimilar (left) to conventional generic drug (right):

BiosimilarsLarge and highly complex biomolecules produced by living cells
Conventional generic drugsRelatively small, simple molecules produced through chemical synthesis
Development and production of biosimilar medicines
Because of their size and structural complexity, and their production using living cell systems, biosimilars require very significant time, effort and expertise, both in their development and in their subsequent production.
The central principle in the development of any biosimilar medicine is its high similarity with an established reference biopharmaceutical. To achieve regulatory approval, the producer of the biosimilar must conclusively demonstrate that its quality, safety and efficacy are entirely comparable to the reference drug.
The time it takes to complete the development of a biosimilar is, on average, seven to eight years. Their development involves a series of stages which are both necessary and complex. Because of the great challenges involved in developing and producing Biosimilar medicines, there are only a very limited number of companies in the world with the know-how and capabilities to develop and produce these new-generation medicines, particularly when it comes to meeting the strict regulatory standards of the world’s highly regulated markets.
The development of a new biosimilar medicine involves five key phases
The regulatory approval process for biosimilar medicines
Approvals for Formycon’s biosimilars are generally made by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA). Both agencies subject biosimilar medicines to a throrough scientific assessment of quality, safety and efficacy before being released by the European Commission for commercialization in the European-, or by FDA for the US market. In Europe, the first Biosimilar was approved in 2006 and in the US in 2015.
Biosimilar medicines require a far greater investment of time and effort to gain regulatory approval than conventional generic drugs. To attain regulatory approval, the producer of the biosimilar must conclusively demonstrate that the quality, safety and efficacy of the biosimilar are highly similar to that of the reference biopharmaceutical. These high standards are attained through intensive analytical testing, clinical trials, and state-of-the-art production processes.
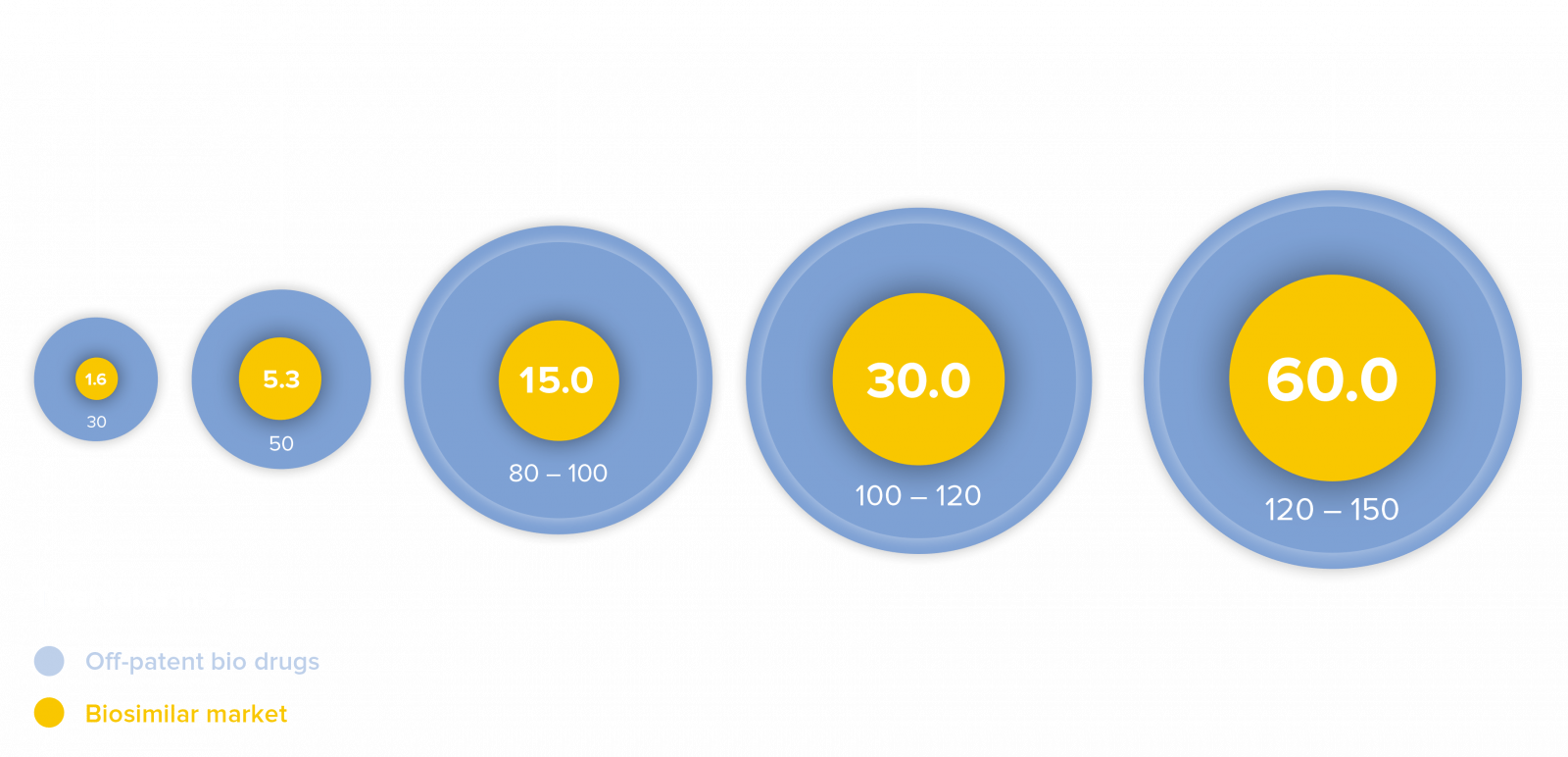
The market for biosimilars
Biosimilar medicines are currently the most rapidly growing segment of the world’s pharmaceutical market. Analysts estimate that, by the year 2025, biopharmaceutical drugs with combined annual revenue between USD 100 – 120 billion will lose their term of patent. The global market for biosimilar medicines, currently estimated at approx. USD 15 billion, could grow to roughly USD 30 billion by the year 2025 and USD 60 billion by the year 2030, in the view of industry experts.
While biopharmaceuticals are remarkably effective at treating serious diseases, they are generally also very costly, posing a financial burden for the healthcare systems even of wealthy developed countries. Because biosimilar medicines offer a Treatment alternative to doctors, pharmacists and patients, thereby creating new competition in the drug market, they help to reduce the costs to healthcare systems while also making these highly effective drug treatments available to more patients.
More information may be found in various sources, such as the Handbook of Biosimilars (Handbuch Biosimilars) published by the Arbeitsgemeinschaft Pro Biosimilars (available in German only).