FYB202
Ustekinumab Biosimilar
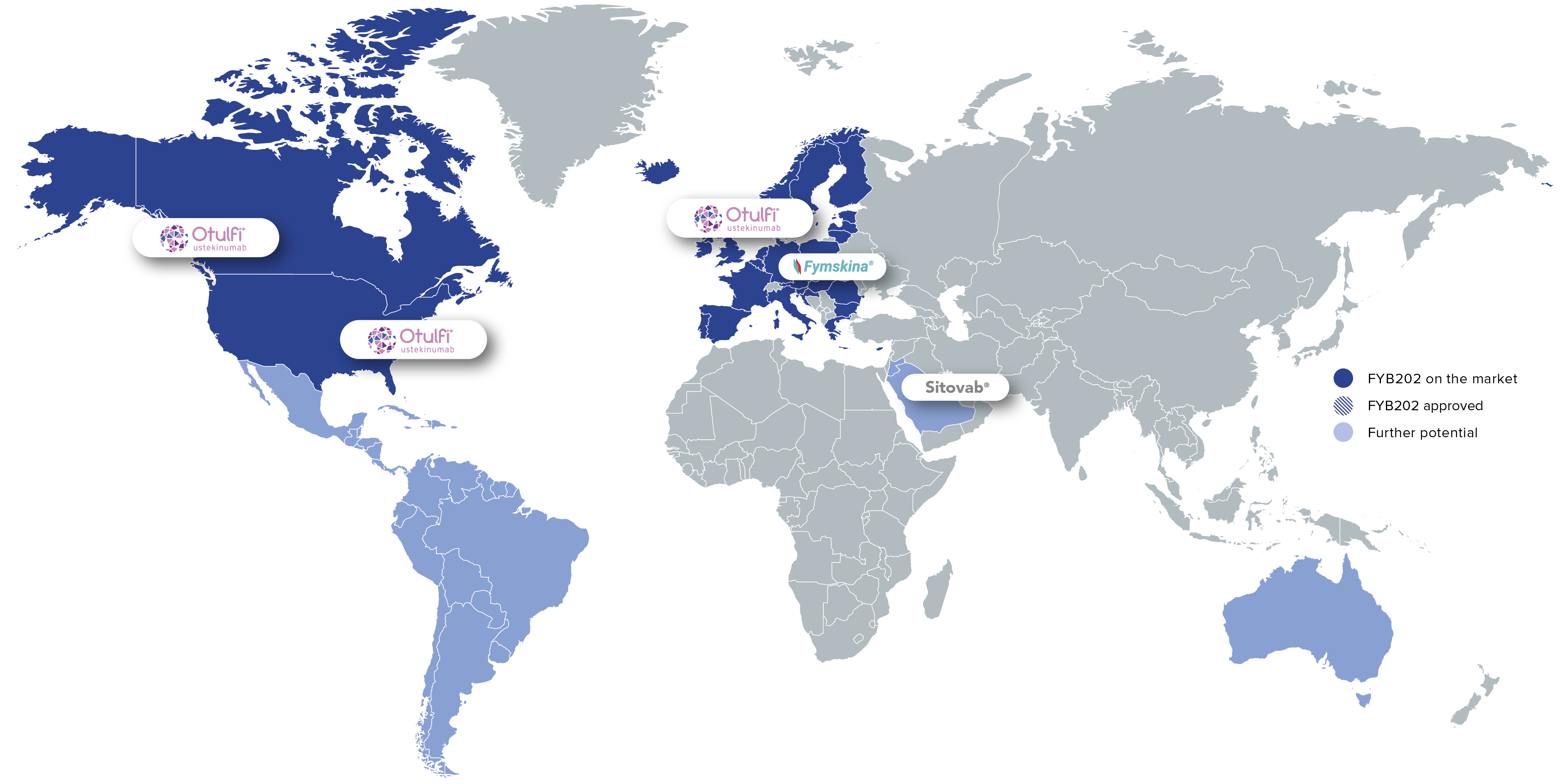
As a treatment option for patients with chronic inflammatory diseases, FYB202 is on the market in the US, Europe, and Canada. Further product launches are planned, including in the MENA region.
Indication Area
Immunology
Active Ingredient Group
Immunosuppressants /
Interleukin inhibitors
Indications of the Reference Drug
Crohn’s disease, ulcerative colitis, plaque psoriasis, psoriatic arthritis*
Market Launch
since Q1/2025
Ustekinumab Market
Global sales of the reference drug Stelara® amounted to approximately US$10.4 billion in 2024. The potential use of ustekinumab for additional therapeutic indications offers further sales opportunities.
Commercialization partners:
![]()
Brand: Otulfi®
Region: Key global markets
![]()
Brand: Fymskina®
Region: Germany
![]()
Brand: Sitovab®
Region: MENA
provided by the EMA or FDA.

FYB202 Biosimilar Development

FYB202 Biosimilar Development
How does FYB202 (Ustekinumab) work?
Ustekinumab is a human monoclonal antibody that targets the cytokines interleukin-12 and interleukin-23 and is used to treat various serious inflammatory diseases such as moderate to severe psoriasis. In 2016, its therapeutic range was expanded to include the treatment of Crohn's disease and, in 2019, ulcerative colitis, both of which are chronic inflammatory bowel diseases. The drug is also used to treat psoriatic arthritis.
June 2025
![]()
Teva subsidiary Ratiopharm becomes secondary commercialization partner for FYB202/Fymskina® in Germany
May 2025
![]()
Commercial Launch of FYB202/Otulfi® in Canada
[Further Information]
March 2025
![]()
Commercial Launch of FYB202/Otulfi® in the U.S. and the E.U.
[Further Information]
January 2025
![]()
Approval in the UK
[Further Information]
January 2025
![]()
Approval in Canada
[Further Information]
December 2024
![]()
MS Pharma becomes commercialization partner for the MENA Region
[Further Information]
September 2024
![]()
FYB202 Approval in the U.S.
[Further Information]
September 2024
![]()
FYB202 Approval in the E.U.
[Further Information]
July 2024
![]()
Positive CHMP opinion for the E.U. marketing authorization of FYB202
[Further Information]
March 2024
![]()
Settlement agreement with Johnson & Johnson for Europa and Canada
[Further Information]
November 2023
![]()
File acceptance by the U.S. Food and Drug Administration (FDA)
[Further Information]
September 2023
![]()
Acceptance of the Marketing Authorization Application by the EMA
[Further Information]
August 2023
![]()
Settlement agreement with Johnson & Johnson for FYB202 in the U.S.
[Further Information]
April 2023
![]()
Successful completion of clinical development of FYB202
[Further Information]
February 2023
![]()
Global commercialization partnership with Fresenius Kabi
[Further Information]
August 2022
![]()
Positive interim results from Phase III study
[Further Information]
March 2022
![]()
Formycon acquires full rights of FYB202 in a transaction with ATHOS KG
[Further Information]
November 2020
![]()
Start of clinical Phase III Study
[Further Information]
October 2019
![]()
Start of clinical Phase I Study
[Further Information]
December 2017
![]()
Formycon and Aristo Pharma establish joint venture for the development of FYB202
[Further Information]
May 2017
![]()
Disclosure of the reference for FYB202
[Further Information]
Preclinical ans clinical Data
Data on the comparability of the biosimilar FYB202 with the reference drug were published in several poster presentations and in a scientific article.
Otulfi® is a registered trademark of Fresenius Kabi Deutschland GmbH in selected countries



