FYB201
Ranibizumab Biosimilar
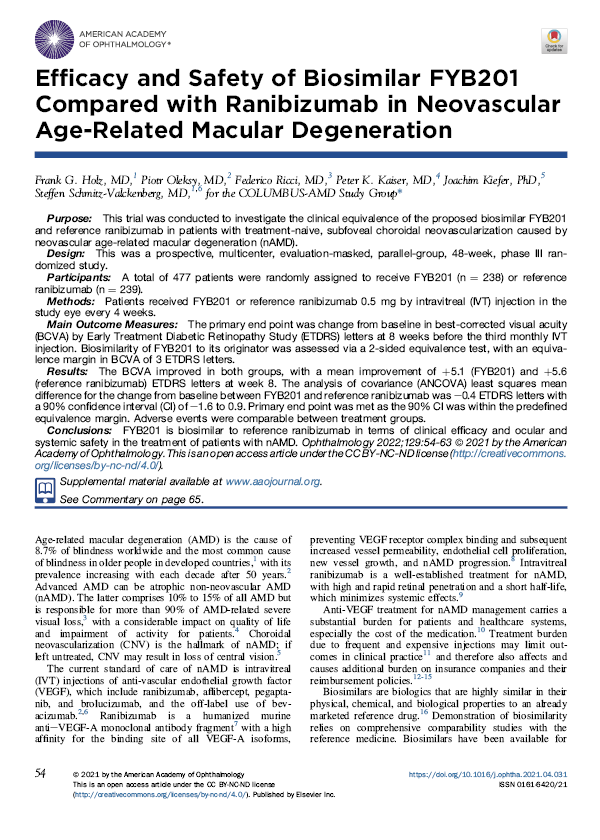
The Lucentis® Biosimilar FYB201 is currently available as a treatment option for patients with severe retinal diseases in 24 countries worldwide. Additional product launches – including in Latin America and on the African continent – are to follow.
Indication Area
Ophthalmology
Active Ingredient Group
VEGF inhibitors
Indications of the Reference Drug
Neovascular (“wet”) age-related macular degeneration (nAMD), Diabetic macular edema (DME), Choroidal neovascularization (CNV), Proliferative diabetic retinopathy (PDR), Macular edema following retinal vein occlusion (RVO)*
Market Launch
since 2022
Business Modell:
50% Formycon project via the stake in Bioeq AG, a joint venture of Formycon AG and Polpharma Biologics Group B.V.
Ranibizumab Market
Ranibizumab is among the most widely usedestablished anti-VEGFs today. In 2024, Lucentis® generated global sales of around USD 1.0 billion.
Commercialization partners:
![]()
Brands: Ranivisio®, Ongavia®, Ranopto®
Region: EU, UK, Canada
![]()
Brands: Cimerli®, Epruvy®
Region: US, Germany
![]()
Brands: Ravegza®, Uptera®
Region: MENA
![]()
Brands: BioUcenta
Region: Sub-saharan Afrika
![]()
Brands: Ranivisio®
Region: Brazil
provided by the EMA or FDA.
FYB201 Biosimilar Development
FYB201 Biosimilar Development
This is how FYB201 (ranibizumab) works
FYB201/Ranibizumab is a monoclonal antibody that is applied to treat adults with visual impairment due to damage to the retina, particularly the macula. The macula is important for the vision needed for everyday activities such as driving, reading and recognizing faces.
The antibody binds to vascular endothelial growth factor A (VEGF-A), which triggers the growth of blood vessels and leads to the leakage of fluid and blood. This is the cause of severe damage to the macula. By blocking VEGF-A, ranibizumab reduces the growth of blood vessels, fluid leakage and swelling.
October 2025
![]()
Ranivisio® is the first Lucentis® biosimilar in Europe to be offered as a pre-filled syringe
[Further Information]
July 2025
![]()
Bio Usawa becomes partner for the commercialization of FYB201/BioUcenta™ across Sub-Saharan Africa
[Further Information]
June 2025
![]()
Approval in Brazil
[Further Information]
April 2024
![]()
Commercial Launch in Canada and Switzerland
[Further Information]
March 2024
![]()
Approvals and Launches in the MENA Region
[Further Information]
March 2024
![]()
Sandoz Inc. replaces Coherus BioSciences as commercialization partner for the US
January 2024
![]()
FYB201/CIMERLI® achieved a market share of 38% in the United States in December 2023
[Further Information]
December 2023
![]()
Approval in Canada
[Further Information]
October 2023
![]()
FYB201/CIMERLI® achieved 25% market share in the United States
[Further Information]
August 2022
![]()
FYB201 approved by the European Commission
[Further Information]
August 2022
![]()
FYB201 approved by the FDA as interchangeable biosimilar
[Further Information]
June 2022
![]()
FYB201 receives CHMP recommendation from EMA
[Further Information]
May 2022
![]()
Approval in UK
[Further Information]
May 2022
![]()
Transaction with ATHOS KG to acquire the biosimilar assets FYB201 and FYB202
[Further Information]
December 2021
![]()
MS Pharma becomes Partner for the Commercialization of FYB201 in the MENA Region
[Further Information]
October 2021
![]()
FDA accepts the biologics license application (BLA) for FYB201
[Further Information]
August 2021
![]()
Submission of the biologics license application (BLA) to the U.S. Food and Drug Administration (FDA)
[Further Information]
June 2021
![]()
Submission of the marketing authorization application to the European Medicines Agency (EMA)
[Further Information]
June 2021
![]()
Teva becomes strategic partner for the commercialization of FYB201 in Europe, Canada, Israel and New Zealand
[Further Information]
November 2019
![]()
Coherus BioSciences becomes Commercialization Partner for the U.S.
[Further Information]
May 2018
![]()
Successful completion of Phase III study
[Further Information]
February 2016
![]()
Start of clinical Phase III study (First Patient-In)
[Further Information]
March 2015
![]()
Successful GMP inspection in Martinsried
[Further Information]
Ranivisio® is a registered trademark of Bioeq AG · Ranopto™ is a trademark of Teva Canada Limited
Ravegza® and Uptera® are registered trademarks of MS Pharma