FYB208
Dupilumab Biosimilar Candidate
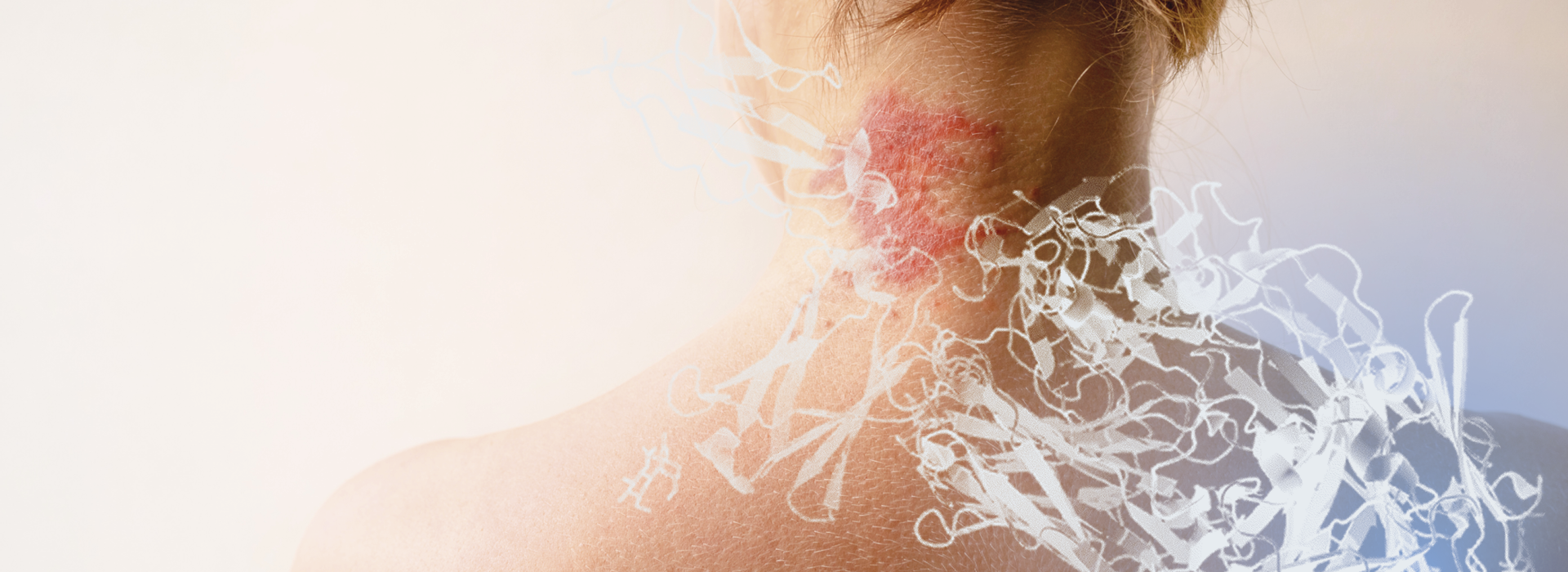
FYB208 is a biosimilar candidate for Dupixent® (dupilumab). Dupixent® is used in immunology to treat various diseases associated with type 2 inflammation. These include atopic dermatitis and asthma.
development progress
Indication Area
Immunology
Active Ingredient Group
Interleukin Inhibitors
Indikations of the Reference Drug
Moderate to severe atopic dermatitis, severe asthma, chronic rhinosinusitis with nasal polyps, chronic obstructive pulmonary disease (COPD)*
Business Modell:
100 % Formycon Project
Market Launch
in the United States and the EU after loss of exclusivity of the reference drug
Dupilumab Market
Due to its convenient administration as a subcutaneous injection, good tolerability, efficacy, and broad range of indications, double-digit growth is forecast for the dupilumab market in the coming years. Sales of around US$14 billion are recorded for 2024. Compared to the previous year, this represents an increase of 22%.1
provided by the EMA or FDA.
FYB208 Biosimilar Development

FYB208 Biosimilar Development
How does Dupilumab work?
Dupilumab is a monoclonal antibody that inhibits signaling by interleukin-4 (IL-4) and interleukin-13 (IL-13) receptors. Thereby, the active ingredient reduces the inflammatory processes and symptoms that occur in atopic dermatitis, certain forms of asthma and COPD, as well as other diseases that are associated with high concentrations of IL-4 and IL-13.
November 2025
![]()
Technical Proof of Similarity (TPoS)
[Further information]
Dupixent® is a registered trademark of Sanofi Biotechnology