FYB203
Aflibercept Biosimilar
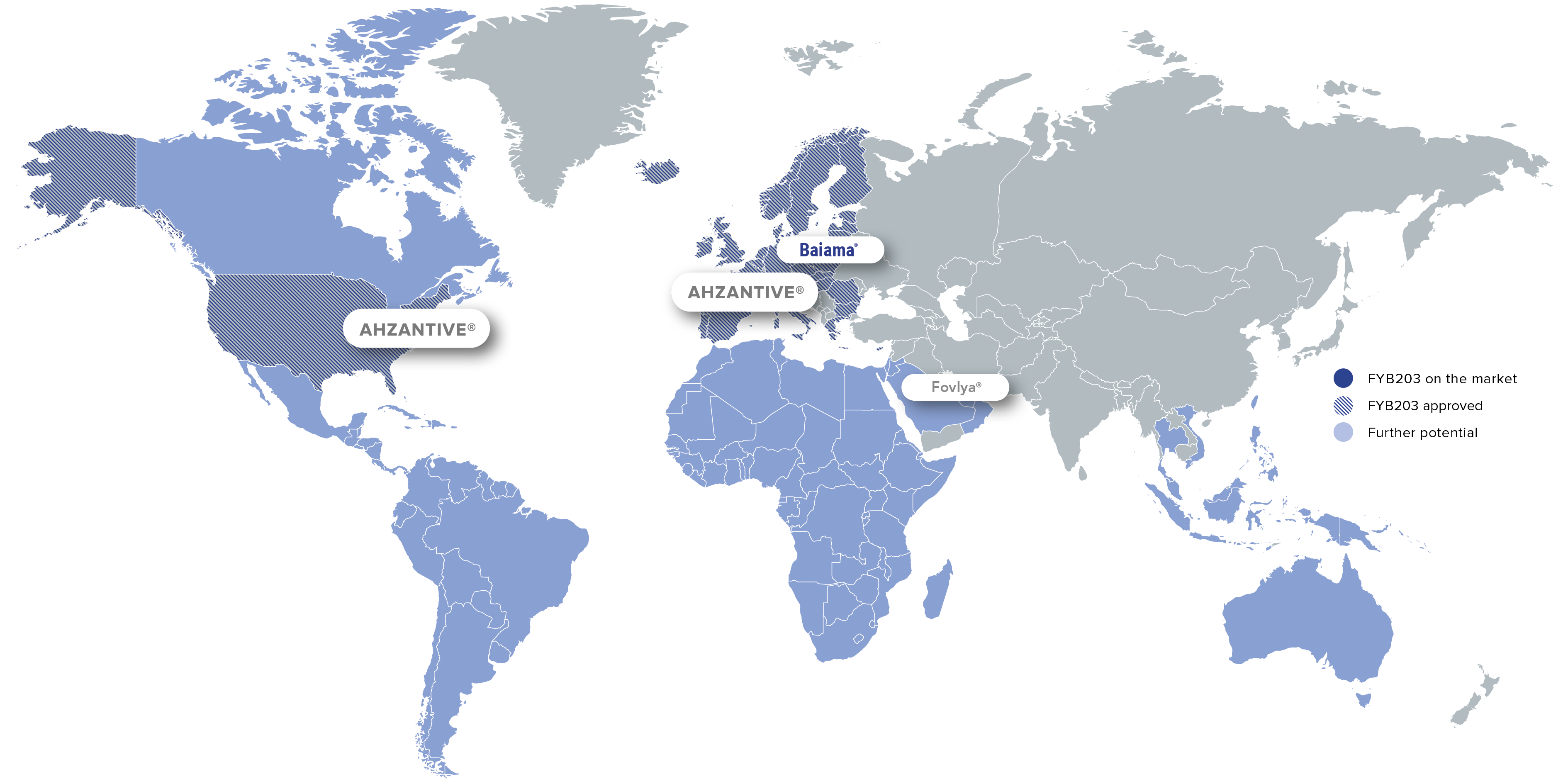
FYB203 is approved in the U.S. and Europe for the treatment of severe retinal diseases such as neovascular (wet) age-related macular degeneration. Market launch in the U.S. is expected in the fourth quarter of 2026.
Indication Area
Ophthalmology
Active Ingredient Group
VEGF Inhibitor
Indications of the Reference Drug
Neovascular (“wet”) age-related macular degeneration (nAMD), Diabetic macular edema (DME), Choroidal neovascularization (CNV), Proliferative diabetic retinopathy (PDR), Macular edema following retinal vein occlusion (RVO)*
Markt Launch
in the U.S. in Q4/2026; in other regions after loss of exclusivity of the reference drug
Aflibercept Market
Aflibercept and ranibizumab together account for more than 90% of the global market for anti-VEGF therapies. In 2024, the reference drug Eylea® in dosages of 2mg and 8mg (high dose) together generated sales of around US$9.5 billion.
Commercialization partners:
![]()
Brand: Ahzantive®
Region: Major Parts of Europa
![]()
Brand: Baiama®
Region: Selected European Countries
![]()
Brand: Ahzantive®
Region: US, Canada
![]()
Brand: Baiama®
Region: Italy
![]()
Brand: Fovlya®
Region: MENA
![]()
Brand: N.N.
Region: Latin America
![]()
Brand: N.N.
Region: APAC
![]()
Brand: N.N.
Region: Australia
provided by the EMA or FDA.

FYB203 Biosimilar Development

FYB203 Biosimilar Development
How does FYB203 (Aflibercept) work?
Aflibercept is a human recombinant fusion protein that binds to both vascular endothelial growth factor (VEGF-A) and placental growth factor (PLGF). Aflibercept thus suppresses the formation of blood vessels in the retina that impair vision. Like Ranibizumab, Aflibercept is injected directly into the vitreous body of the eye.
Due to their different modes of action, aflibercept and ranibizumab complement each other very well in practice. Some patients show a better response to aflibercept, while others are more responsive to ranibizumab. Our biosimilars are designed to improve access to high-quality and more cost-effective treatment options for ophthalmic surgeons and patients.
November 2025
![]()
NTC becomes commercialization partner for FYB203/Baiama® in Italy
[Further Information]
October 2025
![]()
Commercialization partnerships for Australia and Latin America with Actor and Megalabs
[Further Information]
October 2025
![]()
Settlement agreement with Regeneron secures U.S. license date in Q4/2026
[Further Information]
September 2025
![]()
Horus Pharma becomes additional commercialization partner in selected European countries
[Further Information]
June 2025
![]()
US biosimilar specialist Valorum Biologics becomes commercialzation partner for the U.S. and Canada
[Further Information]
February 2025
![]()
Approval of FYB203 in the UK
[Further Information]
February 2025
![]()
Lotus Pharmaceutical becomes commercialization partner for the APAC region
[Further Information]
January 2025
![]()
FYB203 approved by the European Commission
[Further Information]
January 2025
![]()
Teva becomes commercialization partner for FYB203 in major parts of Europe and Israel
[Further Information]
November 2024
![]()
FYB203 receives positive CHMP opinion for the EU marketing authorization
[Further Information]
July 2024
![]()
U.S. approval by the FDA
[Further Information]
May 2024
![]()
MS Pharma becomes commercialization partner for the MENA region
[Further Information]
December 2023
![]()
EMA acceptance of the MAA for FYB203
[Further Information]
November 2023
![]()
EMA submission for FYB203
[Further Information]
August 2023
![]()
File Acceptance for FYB203 by the U.S. Food and Drug Administration (FDA)
[Further Information]
June 2023
![]()
Submission of the biologics license application for FYB203 to the FDA
[Further Information]
February 2023
![]()
FYB203 shows comparable efficacy to the reference product Eylea® in Phase III Study
[Further Information]
August 2020
![]()
Start of the clinical development
[Further Information]
February 2016
![]()
Formycon discloses details regarding the biosimilar candidate FYB203
[Further Information]
May 2015
![]()
Out-licensing of FYB203 to Santo Holding GmbH
[Further Information]
Ahzantive® is a registered trademark of Klinge Biopharma GmbH


